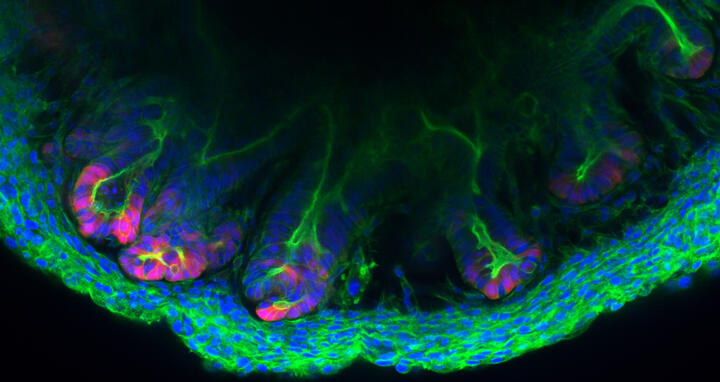
Colon tissue made in the lab
The inner wall of the large intestine, also called the colon, is anything but flat. To increase its surface area, high folds known as “haustra” alternate with deep valleys known as “crypts” – much like a mountain range. The various epithelial cells that cover these mountains and valleys follow a strict architectural pattern. At the very base of the crypts are the stem cells that divide and differentiate into cells that perform functions like producing mucus or reabsorbing water and nutrients. These cells are embedded in the stroma – cell-rich connective tissue that contains a web of extremely fine blood vessels. “We have now succeeded in replicating these crypts outside the body with a level of precision that has not previously been possible,” says Professor Michael Sigal, who heads the Gastrointestinal Barrier, Regeneration and Carcinogenesis Lab at the Berlin Institute for Medical Systems Biology of the Max Delbrück Center (MDC-BIMSB).
Closely mirroring the living organism
Together with researchers at Charité – Universitätsmedizin Berlin, where Sigal also works as a professor and senior physician in gastroenterology, he and his team have created tiny structures called assembloids in a petri dish that contain both epithelial and stromal cells. “The cells are self-organizing and take on almost the same three-dimensional structure as they do in the living organism,” Sigal explains. The results of the collaborative Berlin-based research have been published in the journal "Nature Communications".
An assembloid of a colon created in the lab of Michael Sigal.
“Using our assembloids, we were able to track how the different cell types communicate with one other and help maintain each other’s functions,” explains the study’s lead author Dr. Manqiang Lin, a member of Sigal’s research group. “For example, the stromal cells produce growth factors that influence stem cell division and differentiation at the very base of the crypt.” The researchers pinpointed a group of growth factors called bone morphogenetic proteins (BMPs) as the key information providers. These are known to be involved in the development of the musculoskeletal system, as well as in the regulation of tissue formation and degradation.
Better understanding gastrointestinal diseases
“In the assembloids, the BMPs trigger differentiation in the epithelial stem cells,” says Lin. “These cells then go on to produce BMPs themselves, which in turn cause the stromal cells to secrete factors that promote further differentiation.” If the researchers had used epithelial or stromal cells in their experiments that did not have functioning BMP docking sites, they would not have been able to create the artificial crypts.
In the recently published study, Sigal and his team used mouse cells. “However, we have already performed the same experiments with human cells and obtained similarly good results,” Sigal reports. He hopes the lab-grown crypts will not only lead to a better understanding of how gut tissue develops and organizes itself: “If we were to grow assembloids using patient-specific cells, this could help us better understand the development of intestinal diseases like ulcerative colitis and colorectal cancer – and enable us to develop cutting-edge therapeutic strategies,” the researcher says. “We can also use our models to trial new drugs, thus helping reduce animal testing.”
Text: Anke Brodmerkel
Further information
Literature
Manqiang Lin et al. (2023): „Establishment of gastrointestinal assembloids to study the interplay between epithelial crypts and their mesenchymal niche“. Nature Communication, DOI: 10.1038/s41467-023-38780-3